Lab 8. Invertebrate Community Diversity
in
Wetlands I: Field
April 09 & 10, 2024
08_biol_200_lab_8.RmdNotes:
Lab this week is outside: dress for field work in clothes that can get wet and dirty. Boots are recommended. Note that some plants at the site may have thorns or stickers.
Bring 1-2 computers per group to lab. The lab room will remain locked while we are in the field.
There is a pre-lab but not a post-lab for this week. You must complete your data entry before leaving lab for the day.
Objectives:
Survey invertebrates in three experimental wetland treatments at the Jones Farm.
Become familiar with identification of common aquatic invertebrates.
Calculate invertebrate species diversity with common diversity indices.
KEY WORDS: wetlands; ecosystem services; plankton; replicate; species richness (S); species di-versity (Shannon Diversity Index, H’); species equitability (Shannon Equitability Index, E)
1 Background
Wetlands provide a variety of ecosystem services, including water filtration, groundwater replenishment, mitigation of flood effects, and providing habitat for many organisms. Wetlands are an important reservoir of biodiversity, with particular features known to correlate with biodiversity support[1]. For example, larger wetlands generally support more species – edge habitats are often sub-optimal for species and there is typically more interior than edge habitat in a larger wetland. Wetland plant diversity is also positively correlated with amount of adjacent forest and distance to roads while being negatively correlated with distance from forest habitat.
When describing the biodiversity of a wetland community, we need to consider ways to estimate biodiversity that account for differing sets and abundances of species in order to facilitate comparison among wetland sites. In many cases, these ecological questions are less concerned with the particular species that inhabit an area, and instead center on more general properties of community structure such as components of species diversity. For many questions, such measures may better illustrate underlying ecological processes of competition, predation, nutrient cycling, or energy flow than would characterizing communities by the particular species that inhabit them.
In this exercise, we will compare the diversity of invertebrate planktonic species among several wetlands sampled from the area around Oberlin and varying in size, adjacent forest, and road proximity. The term ‘invertebrate’ describes animals that do not have backbones or bony skeletons and ‘plankton’ describes a wide variety of organisms that occur in water and cannot swim against a current. As a result of this definition, plankton are not a clade—the group as defined does not represent all of the descendants of a single common ancestor. Instead, group membership is defined by the organism’s ecological niche; members of the group range from species of animals and protozoans to algae, bacteria, and archaea!Today, we’re going to focus on planktonic animals.
The invertebrate plankton communities that inhabit the sampled wetlands were recruited naturally. This means that humans did not intentionally transport the organisms into the wetlands. Instead, they were introduced by natural means; one common way in which this occurs is by invertebrates hitching a ride on the feet of waterbirds that frequently fly between wetlands in the region. Once the invertebrates arrive in a wetland, their persistence may be influenced by available food (many are herbivores) and habitat. If we were completing further studies, we might be interested in determining whether community diversity (of plants, invertebrates, or other types of organisms) influences the functioning of the wetlands, such as rates of nutrient cycling.
Today we will collect data that allow us to examine the following hypothesis by testing predictions based on that hypothesis (we’ll do the analysis next week):
Hypothesis: Landscape features of wetlands influence aquatic invertebrate community diversity and species richness.
Prediction 1: Invertebrate biodiversity measures will increase with total wetland area.
Prediction 2: Invertebrate biodiversity measures will increase with amount of adjacent forest.
Prediction 3: Invertebrate biodiversity measures will decrease with increasing distance to forest habitats.
You should think about this hypothesis and the predictions. Can you think of mechanisms by which these landscape features might influence aquatic invertebrate community diversity? You will return to this question next week, when you analyze the data we collect today.
For the pre-lab, you will think about and practice calculating the measures of biodiversity that we’ll estimate for the data you collect this week. In lab, you will use plankton tow nets to collect invertebrate samples in a standardized fashion from one local wetland. We will then return to the lab and count the number of individuals of each type of planktonic invertebrate in your sample plus samples collected from several local wetlands and calculate three measures of community structure relating to species diversity. Next week, we will visualize and analyze the data collected by the class to test whether the measures differ among the wetlands in keeping with the predictions above.
2 Quantifying community diversity
We will use standard methods to quantify ecological data. Some measures are very straightforward to calculate and to understand. The simplest is S, species richness, defined as the number of species sampled. Species richness can be a useful quick-and-dirty means of assessing community diversity, but you should quickly appreciate that species richness is only one aspect of diversity. Species richness does not allow us to determine relative abundance or whether one or two species dominate the habitat while other species are rare. Although you can scan a column of numbers to get some sense of diversity, this method becomes unwieldy to describe, especially for large data sets. We will use two additional basic measures that allow us to standardize data sets for richness and diversity patterns: the Shannon Diversity Index (H’) and the Shannon Equitability measure (E).
Following are descriptions and an example to help you understand these metrics. We present example calculations to give you a sense of what these measures represent, and then you will do the calculations for your data in lab next week using R.
2A Shannon Diversity Index (H’)
The Shannon Diversity Index weights the species richness (the number of species) by how evenly they are represented in the sample. Thus, a sample with primarily one species and only a few individuals belonging to other species will return a Diversity Index that is lower than a sample with equal numbers of all species. The following equation is the shorthand notation for all of the calculations made for a habitat with ‘S’ species.
\[H' ~ = ~ -\sum_{i=1}^{S} p_i \ln p_i\]
To use this equation, we must calculate the frequency with which each species is observed in the sample (\(p_i\)). To calculate this for each species (\(i\)), you must first calculate and record the total number of individuals of each species and sum them to generate the total number of individuals of all species observed in the sample. The frequency for each species (\(p_i\)) in the total sample is calculated by dividing the number of individuals in species \(i\) by the total number of individuals of all species in the sample.
Next, use your calculator to take the natural log (\(\ln\)) of each \(p_i\) value, and record those values for each species. Then multiply the \(p_i\) value by the \(\ln p_i\) value, and record the result for each species (\(p_i \ln p_i\)). Sum the (\(p_i \ln p_i\)) values across all the species, multiply by \(-1\), and you will have calculated H’ for your sample. The following example works through this process in detail.
Example calculating Shannon Diversity Index (H’)
This example demonstrates the sensitivity of the Shannon Diversity Index (H’) to the relative abundance of each species. Below are data from two different habitats. These data differ in species richness as well as the relative abundance of each species. Follow along with the example and double check the calculations using your calculator. Make sure you understand the order of mathematical operations that are taking place.
| Table 1: Example data on species counts for two communities. | |||||||
| Community | Number of Individuals | ||||||
|---|---|---|---|---|---|---|---|
| Species 1 | Species 2 | Species 3 | Species 4 | Species 5 | Species 6 | Species 7 | |
| A | 20 | 3 | 1 | 1 | 0 | 0 | 0 |
| B | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
Note that for Community A, S = 4, and for Community B, S = 7. Thus, in terms of species richness, Community B has greater diversity.
Now, if we calculate H’ for each community, we see that B is also more diverse in terms of H’:
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
An H’ value for one community is not terribly meaningful. However, it’s useful to compare between different sites. Higher H’ values indicate greater biodiversity, both in number of species and relative abundances. But we can take it a step further. The Shannon Diversity Index is actually measuring the entropy of the distribution—the uncertainty associated with your prediction of which species you will find if you simply sample one individual from the population. The more equal the species are in abundance, the higher the uncertainty and thus the higher the value of H’. In fact, for a given value of H’, or entropy, we can determine how many species of equal abundances we would have to have observed to yield this value of H’. To do this, we simply calculate eH’ , where e is Euler’s number (usually there is a calculator button for ex). What does eH’ represent? It is the effective number of species (ENS) for a community.
To see this, calculate eH’ for the two example populations above. In our example, for Community A, eH’ = e0.69 = 2 and for Community B, eH’ = e1.95 = 7. It’s no surprise that the ENS for Community B is equal to the observed number of species since we also see that all species are equally abundant in Community B. However, in Community A, we observed 4 species but most individuals belonged to species 1, so the four species were not even close to equally abundant. Thus, our ENS value of 2 is less than the 4 species actually observed. How much less? That’s what’s estimated by the Shannon Equitability measure (E)!
2B Shannon Equitability (E)
You may notice that the value of H’ has no limit (nor the value of eH’)—it will continue to increase as the number and evenness of species increases. Thus, H’ itself doesn’t really tell us how close we are to the maximum evenness of species that we could have in the population. To standardize our measure, we can divide eH’ by the number of species present in the population. This will give us E, the Shannon Equitability Index, a value that varies from 0 to 1 where the maximum value of 1 represents a community with equal numbers of all species: E = eH’ /S
To see this, let’s calculate E for the two examples above,
For Community A: E = eH’ /S = 2/4 = 0.50
For Community B: E = eH’ /S = 7/7 = 1.00
From these data, you can see that Community A is only half as even as it could possibly be. And we can more easily compare the evenness of communities A and B, even though they have different numbers of species. Thus, Community A is only half as even as Community B (which is maximally even, having equal abundances of all species).
3 Field Sampling Procedures
3A Field materials for each group
Plankton tow net and collecting tube
Two sample storage containers
Sharpie pen and labeling tape
Squirt bottle full of tap water
3B Field sampling
We will use tow net sampling to collect plankton from the water column in a standardized fashion. A tow net consists of a mesh funnel that is slowly and consistently dragged through the water column (see Figure 1). Water enters the large end of the funnel and passes through the mesh. As the funnel is towed through the water column, organisms that enter the large end of the funnel are concentrated into a collecting tube attached to the small end of the funnel.
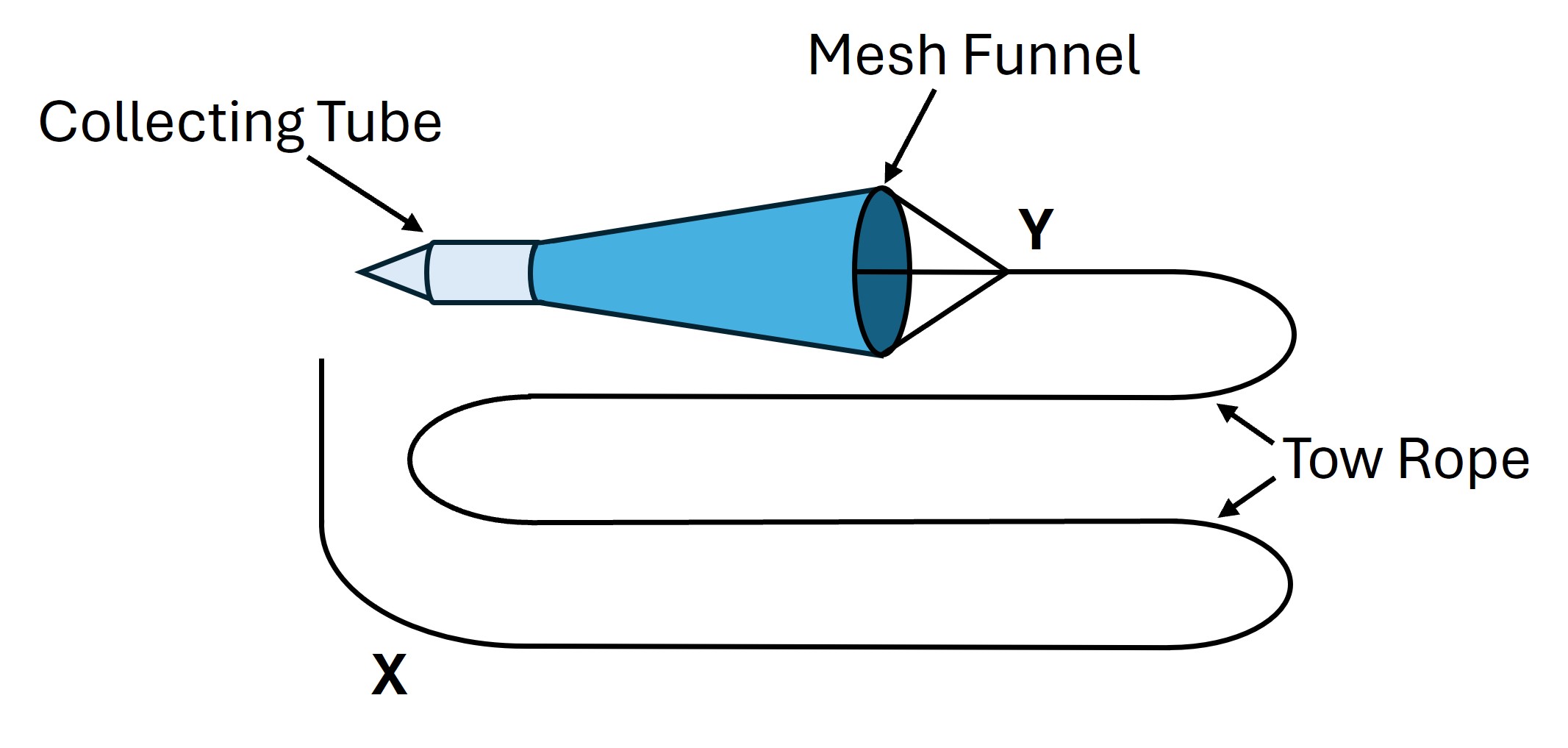
Figure 1: Diagram of tow-net.
X = point to hold
rope when tossing into water;
Y = point on rope at which you stop
towing and remove net from the water.
The volume of water sampled can be estimated by the product of the area of the large end of the funnel and the distance the net is towed. By towing the net through a fixed distance of water, standardized samples can be collected from different water bodies and the resulting data can be converted to numbers of specimens per liter of water. The ropes on the tow nets have two knots (Fig. 1). By holding on to point X on the rope when you toss it into the water, you can ensure that all nets will begin sampling from the same distance from shore. By pulling the rope toward you until you reach the end of the rope, point Y, you can ensure that you tow the net a standardized distance through the water column. Once we get out to the field site, practice a few times before you collect your samples.
When we arrive at the field site, the instructor will demonstrate use of the tow net. Each group should collect a sample from the old reservoir site, taking two samples (replicates). Be sure to label your sample storage containers correctly! Sample two times, with each sampling being done by a different person. Therefore, each group should return to lab with 2 samples; these repeated collections from a single site are replicates. Within your group, figure out who will be collecting each sample. Label your collecting containers and caps with the wetland site ID (MR for Morgan St. Reservoir) - replicate number (1 or 2), the date, and your group name or initials.
Example label: MR-1, 10/31/2017, MCG, HRC, BBK
To collect your tow net samples:
Stand on the edge of the wetland. One person should securely hold the free end of the rope (DO NOT let go) while the person tossing the net holds the rope just above the tow-net attachment point with one hand, keeping the other hand on the marked location X to be sure the two is a standardized length. Toss the net as far as possible from the shore into the water.
Allow the net to gently sink into the water. Do not begin towing it in until the net has completely submerged. The net should be visible but not right at the surface of the water. If you see an air bubble in the net or collecting tube, give the rope a sharp tug or two to force the bubble out. Air bubbles will block sample collection. DO NOT KEEP A SAMPLE THAT HAD AN AIR BUBBLE.
Once the net is completely submerged, slowly and consistently pull the rope toward you to retrieve the net. Be sure to pull slowly, keeping the net fully submerged in the water column. Then, lift the entire apparatus out of the water, such that you minimize the amount of additional water that enters the funnel. Avoid getting lots of detritus from the edge of the water into the net.
Hold the net so that the big end of the funnel is facing up and the collecting tube is suspended below. Use a squirt bottle to rinse all of the sample into the vial. Once all material has been washed into the tube, push the collecting tube upward through the net (taking care not to spill its contents) and pour the contents into a storage container (you may have to first remove a wad of duckweed and other macrophyte material from the top of the tube before you can pour its contents into the storage container). Be sure to securely close the container!
Repeat the procedure at the same location for the second replicate.
When you return to the Science Center, take your tow net to the sink and thoroughly rinse the net and collecting tube. Hang them on the pegs above the sink to dry.
4 Lab Procedures
Lab materials for each group:
|
• 1 dissecting microscope per person • 2 squirt bottles with water • 1 wide-mouthed container for sub-sample • 1 plastic pipette per person • 1 waste beaker (for counted samples) • 1 glass petri dish per person |
• 2 invertebrate identification references • 4 data sheets • 2 replicate samples from Morgan St. Reservoir • 1 replicate sample from another wetland (previously collected by instructors) |
After returning to the lab, each group will work to identify the
invertebrate taxa collected in their samples. Microscopic
protozoans, “algae”, and plants or plant parts present in the samples
will not be identified or counted. You are provided with guides
to some common aquatic invertebrates that occur in local wetlands.
We do not have time to count the entire 50-mL sample, so upon returning to the lab your instructor or TA will create a 25-mL subsample for each of your replicates. You will then count all individuals in one sub-sample per replicate.
Pour one of your sub-samples into one or more provided wide-mouthed containers. Record the identifying information for the sample on your datasheet. Identify and record counts for the large macroinvertebrates that are easily seen without a microscope. Ask your instructor or TA if you are unsure which organisms qualify as macroinvertebrates. Use your microscope to get a closer view of some of the animals.
Then, begin sampling to count up the very small animals—which require use of a microscope to see well. To count these tiny invertebrates, briefly swirl your sample container to ensure everything is in suspension. Use the plastic pipet provided to aliquot samples from the original onto a petri dish, taking care to create 15-18 separate drops of water. This arrangement will allow you to count the organisms more easily by keeping them in one field of view.
Under a dissecting microscope, systematically inspect the sub-sample drops using the technique illustrated in Figure 2. If you use light from below, try to move rapidly through the petri dish as back-lighting quickly heats up the sample and kills the organisms. Be sure to ask your instructor if you have trouble using the microscope. Use a tally count to record the number of individuals of each animal species you encounter on the data sheet.
Note: Many organisms will be easily identifiable with the microscope set at low power. However, some may require higher power for identification. Adjust the magnification of your microscope as needed.
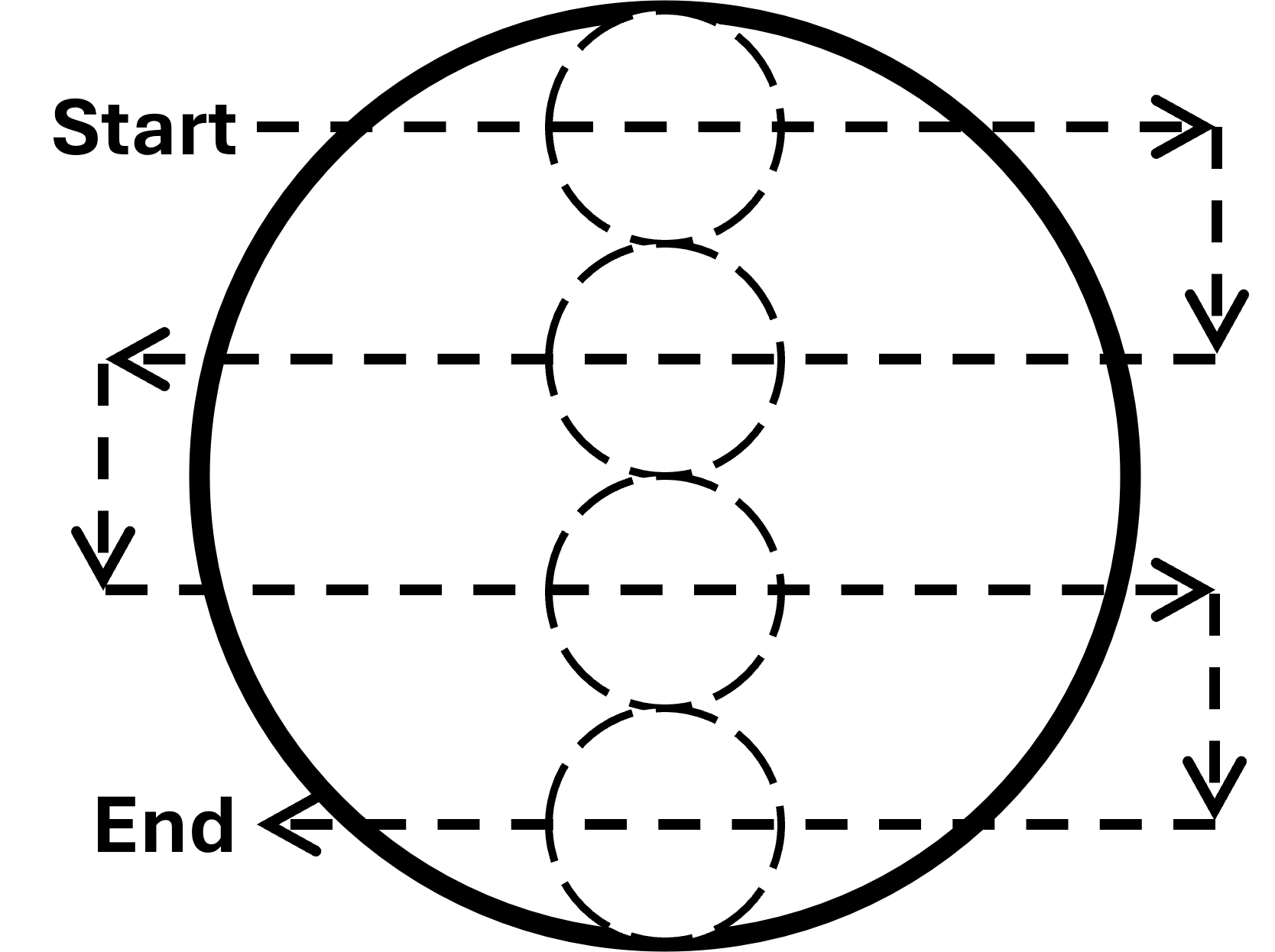
Figure 2: Survey your petri dish systematically to
ensure you cover the entire dish without examining any drops more than
once.
Large solid circle = petri dish; small dashed circles =
field-of-view of microscope (not to scale); dashed arrows = direction of
scan. Start near the top left edge of the dish and scan horizontally in
a straight line to the right until you reach the edge of the dish. Then,
move down the length of one field-of-view and scan to the left. Repeat
this process until you have covered the entire dish.
After identifying all the organisms in your aliquot, dump the contents into a waste beaker, rinse off the petri dish into the waste beaker with a squirt bottle, dry the petri dish, and repeat the steps above until you have gone through your entire 25-mL field sub-sample.
Make sure that all of the data on a data sheet comes from the same replicate sample of a pond. That is, when you finish your first sample, you need to use a new data sheet to record data from a different wetland sample. Each person in a group may use their own data sheet but make sure that before you enter the data into the shared spreadsheet, you combine all of the data for a given replicate from a given pond. For example, there will only be one row of data for the number of Daphnia found in MR-1.
After you have finished with your sample, rinse out your dishes and place them on paper towels on the bench near the sink to dry.
Now, you can enter your data into the shared spreadsheet linked here. Next week, we’ll import everyone’s data into R and calculate species richness, Shannon diversity, and Shannon equitability.
REMEMBER – When you have finished counting and posted your data to the shared spreadsheet please turn in your finished hard copy data sheets to the instructor. There is no post-lab assignment for this lab.
5 Pre-Lab Exercise
Use the lab handout, Section 2 Quantifying community diversity to help you answer the following questions.
Consider a community for which we have the following data on species abundances and complete the table following the instructions from section 2:
| Community C | ||||
|---|---|---|---|---|
| Species | Number of individuals Observed | pi | lnpi | pi lnpi |
| 1 | 21 | |||
| 2 | 20 | |||
| 3 | 19 | |||
| 4 | 14 | |||
| 5 | 13 | |||
| 6 | 13 | |||
| Total = 100 | Total = ______ | |||
- What is the species richness (S) of Community C?
S = _______________
- Calculate the Shannon Diversity Index (H’) for Community C:
H’ = _______________
- Calculate the Shannon Equitability (E) for Community C:
E = _______________
- What do the values of S, H’, and E tell us? How do these metrics differ from each other? (Describe not mathematically but conceptually.)
- Now compare the values of S, H’, and E for Community C with those of Community A (from section 2). Which community has the highest diversity? Explain.