Lab 9. Invertebrate Community Diversity
in
Wetlands II: Data Analysis
April 16 & 17, 2024
09_biol_200_lab_9.RmdNotes:
Please brings a laptop for today’s lab. A loaner from the library will work, we need to use a web browser.
Objectives:
Quantify biodiversity in an ecological system with standardized indices.
Statistically analyze and visually display our data to test for an association of wetland characteristics with biodiversity.
Interpret the results of our analyses to make inferences about local wetlands.
KEY WORDS: Shannon Diversity Index (H’); Shannon Equitability measure (E); barplot; Kruskal-Wallis test; ANOVA (Analysis of Variance)
1 Background – Analyzing Our Data
Last lab period, we collected and quantified invertebrates from several local wetlands that varied in size and amount of adjacent forest. We also have data for the same sites from last fall. For each sample, we identified the invertebrates and calculated diversity indices. This week, we will use the diversity indices to determine if invertebrate diversity varies signficantly among the sites or between seasons. The fall samples were collected in early November, when invertebrates are winding down their activity in preparation for winter. Thus, we might expect spring samples to have higher biodiversity as invertebrates hatch out and grow under conditions of plentiful food. To do so, we will employ two methods of statistical analysis: ANOVA and the Kruskal-Wallis test. Both tests address whether our measures of diversity vary significantly based on our explanatory variables (site, season, land use, wetland size). To refresh your memory, our hypothesis and the specific predictions are:
Hypothesis: Natural features of wetlands influence aquatic invertebrate community diversity and species richness.
Prediction 1: Invertebrate biodiversity measures will increase with total wetland area.
Prediction 2: Invertebrate biodiversity measures will increase with amount of adjacent forest (correlating with land use).
Prediction 3: Invertebrate biodiversity measures will be higher for spring samples compared to fall.
2 Importing Data and Calculating Diversity Estimates
Before we can begin any analysis, we need to import the file of raw data, with the counts of each species in each sample. While you also calculated the diversity indices last week, here we’re going to see how to use R to calculate them, creating a new dataset object with all the diversity measures (S, H’, and E) that we will use as our response variables in the analysis.
YOU SHOULD REFER TO THE HANDOUT (OR YOUR SCRIPT) FROM THE LAB 7 HANDOUT FOR ANY STEPS YOU DO NOT REMEMBER HOW TO COMPLETE.
-
To start, download the datafile from using the link below. The link will downloand a zipped folder that will contain the datafile. This file (named
lab9dataspring2024.csv) contains all of the data collected by all lab groups for all samples in both fall and spring as a CSV (comma-separated values) text file. Make note of where you save the file! - Log into RStudio at https://rstudio.oberlin.edu/. Upload that CSV file into RStudio (hint: Files pane, click Upload).
-
Load the
tidyversepackage so we can use its functions: -
Now, assign the file to a new object so we can use it in R (see below):
inverts <- read.csv("lab9dataspring2024.csv") # assign the file to the object dataRemember that if you uploaded the data file into a folder you will need to put the folder name and a ‘/’ before the datafile name, which will look something like
"Lab 9 Folder/lab9dataspring2024.csv", if the data was uploaded into a folder called “Lab 9 Folder”.Now check out the data, does everything look as it should? You can do this in a number of ways:
inverts # print the object 'inverts' to the screen View(inverts) # shows data in a new tab str(inverts) # show the structure of the object 'inverts'## 'data.frame': 314 obs. of 12 variables: ## $ team : int 2 2 2 2 2 4 4 4 4 4 ... ## $ date : chr "10/31/23" "10/31/23" "10/31/23" "10/31/23" ... ## $ site : chr "duck.pond" "duck.pond" "duck.pond" "duck.pond" ... ## $ taxon : chr "copepod" "ostracod" "daphnia" "rotifer" ... ## $ count : int 117 51 43 14 3 37 4 10 11 36 ... ## $ lab : chr "T" "T" "T" "T" ... ## $ mls : int 25 25 25 25 25 25 25 25 25 25 ... ## $ count.per.ml: num 4.68 2.04 1.72 0.56 0.12 1.48 0.16 0.4 0.44 1.44 ... ## $ sampleID : chr "fall.duck.pond.T.2" "fall.duck.pond.T.2" "fall.duck.pond.T.2" "fall.duck.pond.T.2" ... ## $ hectares : num 0.892 0.892 0.892 0.892 0.892 0.892 0.892 0.892 0.892 0.892 ... ## $ landuse : chr "natural area" "natural area" "natural area" "natural area" ... ## $ season : chr "fall" "fall" "fall" "fall" ...
Remember that in this study we are not examining individual species that are found in the wetland samples. Rather, we are interested in the overall diversity of the community—not which taxa are present but how many and in what relative abundances? That means that when we calculate our diversity measures, we’ll have one value of each diversity estimate for each replicate from each site.
Calculating species richness (S)
-
Now we need to create a new dataset to which we’ll add the diversity measures for each sample (identified by
sampleID). We’ll use the group by and summarize functions to calculate species richness. Notice that we’ll create the new objectdiversityto save the results then we’ll print thediversityobject to the screen so we can make sure it worked correctly.diversity <- inverts %>% group_by(sampleID, site, team, lab, hectares, landuse, season) %>% summarize(S = n_distinct(taxon)) %>% ungroup() # calculate species richness for each sampleID diversity # print the new object to the screen so we can be sure it worked## # A tibble: 6 × 8 ## sampleID site team lab hectares landuse season S ## <chr> <chr> <int> <chr> <dbl> <chr> <chr> <int> ## 1 fall.duck.pond.R.1 duck.pond 1 R 0.892 natural area fall 4 ## 2 fall.duck.pond.R.2 duck.pond 2 R 0.892 natural area fall 9 ## 3 fall.duck.pond.T.2 duck.pond 2 T 0.892 natural area fall 5 ## 4 fall.duck.pond.T.4 duck.pond 4 T 0.892 natural area fall 6 ## 5 fall.duck.pond.W.5 duck.pond 5 W 0.892 natural area fall 6 ## 6 fall.duck.pond.W.6 duck.pond 6 W 0.892 natural area fall 5
In the above code, the
group_byfunction tells R to do the summarize step for each different value of the variables listed. It also means all of those variables will appear in our new diversity dataset. The summarize function says to create the new column ‘S’ and then calculate it by counting up how many different (distinct) taxa are listed for each wetland site.
Calculating the Shannon Diversity Index (H’)
-
Our next step is slightly complicated so be sure that you check your work! We’re going to write code that will do all the work of calculating H’ for each sample. Note: The Shannon Diversity Index, H’, is typically represented by H-prime but R will not accept a variable with a quotation mark in its name so we’ll just use H instead.
Remember from last week’s lab that to get H’ we need to calculate
\[H' ~ = ~ -\sum_{i=1}^{S} p_i \ln p_i\]
for each sample. -
First, we’ll create a dataset of the \(pi\) values for each sample using the functions
xtabs()andprop.table(). Thextabscommand reformats our dataset into a table where columns represent the different species and rows represent the different samples (sampleID), thus each table entry is the number of individuals of that species observed in that sample:xtabs(count.per.ml ~ sampleID + taxon, data = inverts) # see what xtabs function doesThe
prop.table()command then converts the values from thextabsobject into proportions within each row, where the rows represent different samples (sampleID). Lastly, we’ll use theround()function to show 4 decimal places for easier reading.# see what the prop.table function does xtabs(count.per.ml ~ sampleID + taxon, data = inverts) %>% prop.table(margin = 1) %>% round(digits = 4) -
Let’s execute those commands in one go and save the results as an object called
data.pi:data.pi <- xtabs(count.per.ml ~ sampleID + taxon, data = inverts) %>% prop.table(margin = 1) %>% round(digits = 4) head(data.pi) # make sure everything looks good!## taxon ## sampleID amphipod chironomid-midge-larvae chironomid.midge.larva ## fall.duck.pond.R.1 0.0154 0.0000 0.0000 ## fall.duck.pond.R.2 0.0127 0.0000 0.0127 ## fall.duck.pond.T.2 0.0000 0.0000 0.0132 ## fall.duck.pond.T.4 0.1010 0.0000 0.0000 ## fall.duck.pond.W.5 0.0000 0.0000 0.0123 ## fall.duck.pond.W.6 0.0000 0.0000 0.0000 ## taxon ## sampleID clam-shrimp clam.shrimp copepod damselfly-nymph ## fall.duck.pond.R.1 0.0000 0.0000 0.7692 0.0000 ## fall.duck.pond.R.2 0.0000 0.0000 0.1772 0.0000 ## fall.duck.pond.T.2 0.0000 0.0000 0.5132 0.0000 ## fall.duck.pond.T.4 0.0000 0.0000 0.1111 0.0000 ## fall.duck.pond.W.5 0.0000 0.0613 0.2454 0.0000 ## fall.duck.pond.W.6 0.0000 0.0000 0.2292 0.0000 ## taxon ## sampleID damselfly.nymph daphnia dragonfly-nymph flatworm isopod ## fall.duck.pond.R.1 0.0000 0.1385 0.0000 0.0000 0.0000 ## fall.duck.pond.R.2 0.0127 0.2911 0.0000 0.0253 0.0127 ## fall.duck.pond.T.2 0.0000 0.1886 0.0000 0.0000 0.0000 ## fall.duck.pond.T.4 0.0000 0.3737 0.0000 0.0000 0.0101 ## fall.duck.pond.W.5 0.0000 0.1595 0.0000 0.0000 0.0000 ## fall.duck.pond.W.6 0.0000 0.3333 0.0000 0.0625 0.0000 ## taxon ## sampleID leech mosquito-larva mosquito.larva nematode ostracod ## fall.duck.pond.R.1 0.0000 0.0000 0.0000 0.0000 0.0769 ## fall.duck.pond.R.2 0.0000 0.0000 0.0000 0.0000 0.4304 ## fall.duck.pond.T.2 0.0000 0.0000 0.0000 0.0000 0.2237 ## fall.duck.pond.T.4 0.0000 0.0000 0.0000 0.0000 0.0404 ## fall.duck.pond.W.5 0.0000 0.0000 0.0000 0.0000 0.1227 ## fall.duck.pond.W.6 0.0000 0.0000 0.0000 0.0000 0.3333 ## taxon ## sampleID rotifer tardigrade water-mite water.mite ## fall.duck.pond.R.1 0.0000 0.0000 0.0000 0.0000 ## fall.duck.pond.R.2 0.0000 0.0000 0.0000 0.0253 ## fall.duck.pond.T.2 0.0614 0.0000 0.0000 0.0000 ## fall.duck.pond.T.4 0.3636 0.0000 0.0000 0.0000 ## fall.duck.pond.W.5 0.3988 0.0000 0.0000 0.0000 ## fall.duck.pond.W.6 0.0417 0.0000 0.0000 0.0000 -
Next, we need to take those pi values and calculate the product \(pi \ln pi\) for each of them, saving this in a new dataset called
data.pilnpi:data.pilnpi <- data.pi*log(data.pi) -
We’re ready to sum up the values for each sample and multiply them by \(−1\) to complete the calculation of H’ for each sample, saving them in a new data frame called
H:H <- data.frame(sampleID = rownames(data.pilnpi), # create new dataset with the H’ values H = round(-1*rowSums(data.pilnpi, na.rm = T), digits=3), row.names = NULL) H # check to see what the results look like!## sampleID H ## 1 fall.duck.pond.R.1 0.737 ## 2 fall.duck.pond.R.2 1.437 ## 3 fall.duck.pond.T.2 1.220 ## 4 fall.duck.pond.T.4 1.387 ## 5 fall.duck.pond.W.5 1.487 ## 6 fall.duck.pond.W.6 1.376 -
Note that this new data frame, H, has the same
sampleIDcolumn as the diversity object we made earlier to store species richness calculations. This means that we can easily merge these two objects so we have both S and H’ in one object:diversity <- merge(diversity, H, by = "sampleID") diversity # check the result!## sampleID site team lab hectares landuse season S H ## 1 fall.duck.pond.R.1 duck.pond 1 R 0.892 natural area fall 4 0.737 ## 2 fall.duck.pond.R.2 duck.pond 2 R 0.892 natural area fall 9 1.437 ## 3 fall.duck.pond.T.2 duck.pond 2 T 0.892 natural area fall 5 1.220 ## 4 fall.duck.pond.T.4 duck.pond 4 T 0.892 natural area fall 6 1.387 ## 5 fall.duck.pond.W.5 duck.pond 5 W 0.892 natural area fall 6 1.487 ## 6 fall.duck.pond.W.6 duck.pond 6 W 0.892 natural area fall 5 1.376
Calculating the Shannon Equitability Index (E)
-
Now we just have one more thing to calculate: species evenness using the Shannon Equitability Index, E. Remember, this measure combines the information from S and H’, like so: \(E = \frac{e^{H'}}{S}\)
This one is pretty straightforward to calculate using our diversity object to create a new column for E:
diversity <- diversity %>% mutate(E = round(exp(H)/S, digits = 3)) # make a new column in diversity that calculates E for each sample diversity # check the result!
Now we are ready to move on to visualizing and then analyzing our diversity dataset!## sampleID site team lab hectares landuse season S H ## 1 fall.duck.pond.R.1 duck.pond 1 R 0.892 natural area fall 4 0.737 ## 2 fall.duck.pond.R.2 duck.pond 2 R 0.892 natural area fall 9 1.437 ## 3 fall.duck.pond.T.2 duck.pond 2 T 0.892 natural area fall 5 1.220 ## 4 fall.duck.pond.T.4 duck.pond 4 T 0.892 natural area fall 6 1.387 ## 5 fall.duck.pond.W.5 duck.pond 5 W 0.892 natural area fall 6 1.487 ## 6 fall.duck.pond.W.6 duck.pond 6 W 0.892 natural area fall 5 1.376 ## E ## 1 0.522 ## 2 0.468 ## 3 0.677 ## 4 0.667 ## 5 0.737 ## 6 0.792
Visualizing the Data
Remember that the first step in data analysis is to make some pictures of the data—see what patterns are in your data. This helps you to interpret your subsequent statistical results, since they should reflect what you can see in the data.
-
You may remember that we have replicate samples for each site (each row of the diversity dataset is a replicate from one site). Just looking at the dataset, you can see any variation among sites or other variables.
diversity## sampleID site team lab hectares landuse season S H ## 1 fall.duck.pond.R.1 duck.pond 1 R 0.892 natural area fall 4 0.737 ## 2 fall.duck.pond.R.2 duck.pond 2 R 0.892 natural area fall 9 1.437 ## 3 fall.duck.pond.T.2 duck.pond 2 T 0.892 natural area fall 5 1.220 ## 4 fall.duck.pond.T.4 duck.pond 4 T 0.892 natural area fall 6 1.387 ## 5 fall.duck.pond.W.5 duck.pond 5 W 0.892 natural area fall 6 1.487 ## 6 fall.duck.pond.W.6 duck.pond 6 W 0.892 natural area fall 5 1.376 ## E ## 1 0.522 ## 2 0.468 ## 3 0.677 ## 4 0.667 ## 5 0.737 ## 6 0.792 -
Let’s make a bar plot to get a visual sense of the variation between replicates. With
ggplot(), we can usegeom_colto make a barplot. We have to specify the x and y variables plus we’ll color the bars by season, which corresponds to fall or spring. Let’s check out our species richness (S) values:diversity %>% ggplot() + geom_col(mapping = aes(x = sampleID, y = S, fill = season)) + coord_flip() + ylab("Species richness (S)") + ggtitle("Species richness of all samples")Each bar represents one water sample and color indicates season. You can see any variation among sites, replicates, or lab days. Here’s another way to show the same data, using points instead of bars:
diversity %>% ggplot() + geom_jitter(mapping = aes(x = site, y = S, col = season), show.legend = T) + theme_bw()Ask yourself: Are there any patterns? Does it look like species richness varies between wetland sites?
These plots are useful to help us see the total variation in our data but it doesn’t really show us whether diversity seems to relate to any of our explanatory variables. That is, this barplot does not help address our hypothesis. Instead, let’s create a box-and-whisker plot (or boxplot). With the box plot, we can graph the data by groups, so that we show the minimum data value, the first quartile (the 25th percentile of the data), the median (the 50th percentile or middle of the data), the 3rd quartile (the 75th percentile), and the maximum data value. This can give us a better sense of whether the groups differ from each other on average. -
For the first boxplot, we’ll color the boxes by site. Then we’ll make a second boxplot that differentiates site and also season.
diversity %>% ggplot() + geom_boxplot(mapping = aes(x = site, y = S, fill = site), show.legend = F) + ylab("Species richness (S)") + ggtitle("Boxplot of species richness for each wetland site") + theme_bw()And here’s the second boxplot, including season:
diversity %>% ggplot() + geom_boxplot(mapping = aes(x = season, y = S)) + ylab("Species richness (S)") + ggtitle("Boxplot of species richness for each season by wetland site") + facet_wrap(~ site) + theme_bw()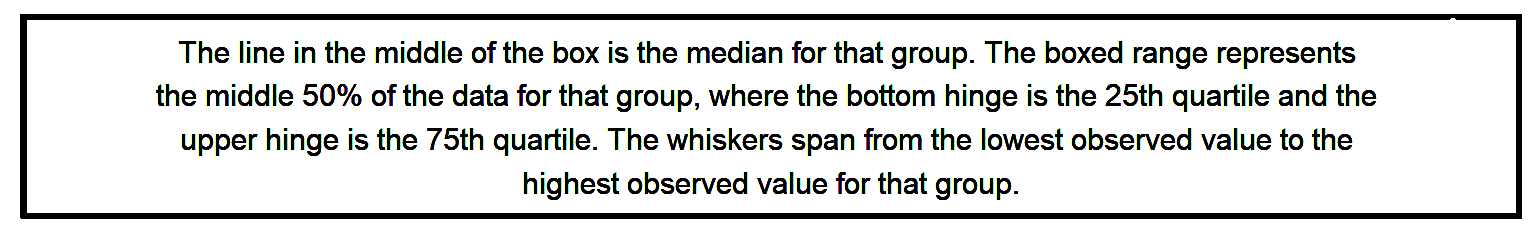
Ask yourself: Does this graph show the same patterns you saw in the bar or dot plots? What’s the benefit of this box plot versus the bar or dot plot? - Now make plots for the other two response variables, H’ and E. Ask yourself: What patterns do see for those variables?
-
We can also look at a summary of our data in table form. We’ll ask R to calculate the mean, standard deviation (sd) and sample size (n) for each diversity measure for each sample, calculating a mean for each season. If we wanted to average by a different variable, like land use, we’d have to change this code slightly.
diversity %>% group_by(site, season) %>% summarize(meanS = mean(S), stdevS = sd(S), meanH = mean(H), stdevH = sd(H), meanE = mean(E), stdevE = sd(E), n = length(S))## `summarise()` has grouped output by 'site'. You can override using the ## `.groups` argument.## # A tibble: 10 × 9 ## # Groups: site [5] ## site season meanS stdevS meanH stdevH meanE stdevE n ## <chr> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <int> ## 1 duck.pond fall 5.83 1.72 1.27 0.278 0.644 0.125 6 ## 2 duck.pond spring 5.25 1.26 1.35 0.218 0.756 0.135 4 ## 3 hwy20 fall 6.33 2.16 0.593 0.416 0.358 0.277 6 ## 4 hwy20 spring 8.67 1.53 1.31 0.238 0.455 0.163 3 ## 5 morgan.big fall 4.88 1.25 0.612 0.408 0.418 0.181 8 ## 6 morgan.big spring 5.17 2.40 1.04 0.543 0.693 0.258 6 ## 7 morgan.small fall 4.86 2.12 0.898 0.230 0.574 0.168 7 ## 8 morgan.small spring 7 2.10 1.39 0.410 0.602 0.128 6 ## 9 oberlin.reservoir fall 5.6 1.67 0.337 0.186 0.275 0.109 5 ## 10 oberlin.reservoir spring 4 2.12 0.865 0.247 0.704 0.249 5With this function, we are able to specify all the functions we want to run (i.e., mean, standard deviation, and sample size) and what grouping variables to use. You could also include other response or explanatory variables and save the output as an object that we could access later (we’ll want it for some of the analyses):
div.summary <- diversity %>% group_by(site, season, landuse) %>% summarize(meanS = mean(S), stdevS = sd(S), meanH = mean(H), stdevH = sd(H), meanE = mean(E), stdevE = sd(E), n = length(S)) View(div.summary)
The table should show the same patterns that you were seeing in your plots earlier.## `summarise()` has grouped output by 'site', 'season'. You can override using ## the `.groups` argument. -
You can also export the summary table as a file that you can then open in Excel or Google Sheets to format it nicely for display. Here’s the code to export the object as a CSV file:
After running that code, you need to find the file you just created in the Files pane, click the box next to the file name, and then click More \(\rightarrow\) Export… Give it a name, click Download, and choose where to save the file on the computer.write_csv(div.summary, file = "diversitysummary.csv", quote = "none")
4 Analyzing the Data
Now that we’ve checked out our data graphically, we can run an analysis to get statistics to place some probabilities on any patterns in our data. Remember from Lab 7 Adaptive Morphology that we determine what kind of plot and analysis to use based on the types of variables we have, as in this table.
| Explanatory Variable | Response Variable | Graph to use | Analysis to use | Values to report |
|---|---|---|---|---|
Continuous |
Continuous |
Scatterplot
( |
Linear regression ( |
Slope, y-intercept, P-value |
Categorical |
Continuous |
Boxplot
( |
ANOVA and Kruskal-Wallis |
P-value for explanatory variable |
Overview of Analysis Methods
Last time, in Lab 7, we ran linear regressions because we had continuous response and explanatory variables. We can do that again if we are using continuous explanatory variables (hectares is continuous, the area of the pond, but lab day, season, and land use are categorical). Refer back to Lab 7 if you need a refresher on how to run a regression and how to understand the results. In other cases, when we have continuous response variables and categorical explanatory variables we’ll run an ANOVA (Analysis of Variance)—this test checks whether two (or more) categories differ in their average responses. The statistical null hypothesis for an ANOVA is that the different groups (for example, fall or spring season) have the same mean for the response variable. The underlying logic is similar to that of the chi-square test we ran in a previous lab exercise: we calculate the overall average and variance of the dataset, lumping all individuals together as a single group (this is our “expected” value) and we compare that mean to the means and variances we calculate within each group (our “observed” values). The bigger the difference between the observed and expected values, the more likely that the groups have different means for the response variable. ANOVA is what’s known as a “parametric” test; that is, the method assumes that the data we are analyzing come from a known probability distribution, in this case the normal distribution. When data are normally distributed, it means that we can accurately describe the variation of the sample population using things like the arithmetic average and the sample variance (an estimate of how spread out the data are).
However, not all data meet the assumptions of normality. When data are not normal (nor well-described by other familiar distributions), we can use different tests, known as non-parametric tests. The Kruskal-Wallis test is a non-parametric test that is kind of like an ANOVA but for data that are not normal. Instead of calculating the mean of S, the Kruskal-Wallis test will order our data values and assign them a rank (e.g., 1 for the smallest value, 2 for the next smallest value, etc). Then, you calculate the average rank and the sum of ranks for each treatment group and the total dataset. The test statistic is then calculated from the sum of squared deviations of the groups relative to the total dataset. The Kruskal-Wallis test statistic is somewhat like a chi-square test statistic (remember observed minus expected squared?) and is in fact chi-square distributed. In the end, you get a P-value for the probability that the ranks differ between the groups.
Which test should we believe? Well, it depends… ANOVA is actually quite robust to deviations from normality, at least certain types of deviations. The Kruskal-Wallis test doesn’t assume the data to be normally distributed, but is less powerful than ANOVA and can be misleading under certain conditions—caution may be warranted if groups do not have 5 or more observations each (this is also true for a chi-square test). In our data, we might be worried about the assumption of normality but we also have relatively few observations, so neither test is a perfect fit for this data (as happens all too often in statistical analysis!). Because this is not a statistics course, we’re not going to go into detail about checking normality (it’s not simple to interpret) or why small numbers of observations can cause problems. Instead, we’re going to run both tests and see if they give us similar results, which is what we expect to happen most of the time. Then it’s up to you to decide how to interpret them!
Side note: If you have more questions or are interested in learning more, you should consider taking STAT 113! In the meantime, there are some good online resources to explore—see http://www.biostathandbook.com/index.html—and your instructors are happy to discuss further.
Analyzing S with ANOVA
-
When we run the ANOVA, we use the full dataset with all the samples and we include site as an explanatory variable. We could also use other explanatory variables at the same time but to start we’ll just use site. The function to run an ANOVA in R is
aov()and as arguments you need to supply a formula (of the y ~ x variety) and the name of the dataset containing variables x and y. We’ll run the function anova on the output ofaov()as that will give us a nice summary of the results. Here’s the code for an ANOVA model of S with site as the explanatory variable:And now to print the summary:
S.site.aov # print the summary results to the screenYou should get results that have the same layout as below but different numbers (the example was run on a different data set from the one you are using):
## Analysis of Variance Table ## ## Response: S ## Df Sum Sq Mean Sq F value Pr(>F) ## site 4 10.776 2.6940 0.8351 0.5148 ## Residuals 27 87.099 3.2259How do we read and interpret this table of information?
In this table, the
Pr(>F)is the P-value, we have one for each explanatory variable we included in the model.The F-value is the test statistic, analogous to the \(\chi^2\) value of the chi-square test.
In this example, the first row of the table tells us about the effect of
siteon species richness. For these results, the probability that sites do not differ, on average, in species richness is P = 0.5148, which is much higher than 0.05. Thus, we would conclude that these different wetlands do not show significant differences in species richness.If you are reporting these results, it would be typical to provide the entire ANOVA table or the F-value, degrees of freedom (Df), and the P-value (written as F4,27 = 0.8351 and P = 0.5148 for the effect of site). However, it is common to present only the P-value when an effect is not significant. For example, in this case we could say “there was no significant difference in species richness among sites (F4,27 = 0.8351, P = 0.5148)”.
-
Now, if we want to include a second explanatory variable, we change the anova code a little bit to include a second variable. Let’s try running the anova with both site and land use.
Notice how
siteis included in the model—in the form a/b—this is read as “b is nested within a”. That means that each sitesitehas a value of species richness for only a singlelanduse. As a result, the effect ofsiteis not independent of the effect oflanduse,siteis nested inlanduse. If we usedseasoninstead, that would not be nested and we would writeS ~ site + seasonfor that part of the code.And now to print the summary:
Referring to the instructions about the first model, how do you think we interpret these results? Keep in mind that the effect of different sites is captured by theS.site.land.aov # print the summary results to the screenlanduse:siteline in the output.
Analyzing S with Kruskal-Wallis
-
Now for the Kruskal-Wallis test! For this one, we can only include a single response variable and a single explanatory variable. We write the Kruskal-Wallis model similar to that of an ANOVA (i.e., y ~ x), only including site and we also provide the dataset name:
S.site.kruskal <- kruskal.test(S ~ site, data = diversity) S.site.kruskal # print the test results to the screenwhich should print out something like (run on a different dataset from the one you are using):
## ## Kruskal-Wallis rank sum test ## ## data: S by site ## Kruskal-Wallis chi-squared = 3.2761, df = 4, p-value = 0.5127Similar to the ANOVA results, you would typically report the test statistic (4 here), the degrees of freedom (df), and the P-value for this test.
Ask yourself: Does the result from the Kruskal-Wallis test agree with that from the ANOVA?
5 Export Your Results
- Remember, to export graphs, you need to save the file to the folder in RStudio, then go to the File panel, click the box to select the graph, and then click More → Export to download the file to your computer.
-
You may want to export your summary table too (you can then open it in another program, like Excel, to make a table that looks nice):
write_csv(div.summary, file = "diversity.table.csv", quote = "none")You can also export the output of the statistical tests this way:
write_csv(data.frame(S.site.aov), file = "S.site.anova.out.csv", quote = "none") write_csv(data.frame(S.site.kruskal[]), file = "S.site.kruskal.out.csv", quote = "none")
6 Pre-lab exercise
Consider the data that we collected last week in answering the following. You may choose to refer to your lab 8 handout too. This exercise is due by the start of your lab period.
- What are our explanatory variables?
- What are our response variables?
- What is our null hypothesis?
- If the results of our statistical tests yield P < 0.05, what does
that tell us about our prediction(s)? About our hypothesis?
- What does it tell us about our hypothesis/predictions if our P-value
is greater than 0.05?
7 Post-Lab Assignment
This exercise is due by the start of next week’s lab.
To answer the questions below, you will need to do some additional analyses beyond what was described in this handout. We showed you how to analyze the effect of site, answering the question ‘Do wetland sites differ in diversity?’ Now, you will need to go back and do the same analyses, for S, H’, an E as response variables but then including either landuse or season as explanatory variables.
Combine informative, nicely formatted graphs and tables into a document with the relevant P-values next to the graphs. Include typed answers to the following questions and turn them in with your figures next week. Support your answers by referring specifically to your figures and/or the results of your tests of significance, where appropriate. We only did part of these analyses in lab, since we went through using wetland site as an explanatory variable. You will need to run additional analyses to check for the effects of adjacent forest (landuse) or seasonality (season).
A single group of up to 4 people may turn in 1 group assignment, assuming all worked together to complete the assignment.
- Consider the analyses described here for wetland site. Do we have
any evidence that these 5 wetlands differ in diversity, as represented
by species richness (S), species diversity (H’), or
species evenness (E)? Explain and comment on each of these
metrics and how they vary among sites.
- Describe the additional analysis you chose to do for landuse or
season (considering all of S, H’, an E). Did
you find any evidence that the diversity metrics differed for this
additional explanatory variable? Explain, being sure to comment on the
output for each diversity measure.
- After considering your results, think about the hypothesis and
predictions we were testing. They don’t really tell us about how or why
wetland size, adjacent forest, or season would affect aquatic
invertebrate diversity. Based in part on your observations and data from
last week, propose a new hypothesis (with a mechanism!) and one or two
predictions that might explain the variation in diversity we have
observed for the community of aquatic invertebrates.
- Other than wetland size, adjacent forest, or season, are there other
comparisons you might make with these data?(Consider other variables
that are in the dataset but that we did not use in our analyses.)
- For these analyses, we only examined planktonic invertebrates that
occurred in the water column sampled all in one specific week at two
times of the year. How might this particular method of sampling
influence the results we obtained?
- What additional data could you collect to compare biodiversity among
wetlands? To answer this question, consider other aspects of the sites
that you might want to evaluate.