Lab 10. Crayfish Population Ecology - Field
April 23 & 24, 2024
10_biol_200_lab_10.RmdNotes:
One member of each group should bring a laptop for data entry upon return to the lab.
Dress for field work: wear clothes and shoes that can get wet and dirty. Boots and long pants are recommended.
Objectives:
Explore primary relationships between soil properties, vegetation, and crayfish burrows.
Examine methods used to study environmental variables in natural communities.
Practice stating hypotheses and predictions.
Compare competing hypotheses for explaining potential mechanism(s) shaping crayfish burrow lo-cations.
Collect field data on soil properties, vegetation type, and crayfish burrows.
Enter data into a spreadsheet in preparation for analysis in Lab 11.
KEY WORDS: crayfish; burrow; transect; plot; vegetation; soil properties; pH; soil moisture; soil nutrient content; plant productivity; burrow; sit-and-wait predation
1 Burrowing Crayfish Background
Crayfish, also known as crawfish or crawdads, are aquatic macroinvertebrates, often found living in surface waters (rivers, ponds) where they burrow under rocks and consume plant material and detritus. While many crayfish live in surface waters, some species burrow down to the water table in much drier, more terrestrial habitats (Figure 1). All crayfish species have some capacity to burrow but primary burrowers spend nearly their entire life cycle in the underground burrow. In a more terrestrial habitat, the burrow provides necessary access to water (crayfish have gills), protection from predators, and access to food.
Dr. Angie Roles and her team of Oberlin student researchers are studying burrowing crayfish found in this area, including right on Oberlin’s campus – in the fields around the solar array. They have identified areas where burrows can be found and are interested in the structure of the burrows and behavior of the crayfish in and out of the burrow. The Roles Lab has identified Creaserinus fodiens, the Digger Crayfish, living in burrows at the solar array fields. These animals excavate burrows that can extend more than 4 feet into the ground and the burrow openings are sometimes marked by a mound of soil, known as a chimney (Figure 1).
 reproduced for educational purposes.](../reference/figures/crayfish_burrow.jpg)
Figure 1: (A) A typical crayfish burrow with tunnels that reach the water table. Mounded earth is common at burrow entrances as crayfish excavate their burrows. (B) An adult Lacunicambarus thomai active during daylight at the entrance of its burrow (entrance not visible due to leaf litter). Figure 1 from Graham et al. (2022) reproduced for educational purposes.
While all crayfish have some capacity to burrow, there is great variation in how much a species relies upon burrows. The species at the solar array fields are primary burrowers, which means they spend nearly their entire life cycle underground. In contrast, crayfish on the opposite spectrum of the classification (tertiary burrowers), spend their lives mostly aboveground, usually in surface waters (streams and rivers), and may only burrow to escape frost, or to shelter during dry conditions. In all forms of burrowers (primary, secondary and tertiary), groundwater depth is influential to their inclination to burrow. Floodplains with shallow water tables (e.g., poorly draining soil) typically support greater amounts of crayfish burrows (Bearden et al. 2022). For some species, sedges, grass-like monocots that grow in moist soils, have been found to be positively correlated to burrow activity (Rhoden et al. 2016) . In contrast, woody vegetation has been negatively correlated to crayfish burrows for some species, causing authors to suggest that crayfish require (and are adapted to) open, unshaded vegetation habitats (Bloomer et al. 2022; Adams et al. 2021). Bloomer et al. (2022) studied Creaserinus fodiens and found higher burrow densities in mowed habitats compared to unmanaged. However, very few studies have explored the environmental requirements of burrowing crayfish species and it is unclear how much these results generalize across species.
Primary burrowers are challenging to study because of their subterranean lifestyle. They are believed to eat, reproduce, and nearly always remain belowground, coming to the surface when the water table is high to forage, hunt, or remove soil during excavation and rarely emerging from burrows during daylight hours. While crayfish do forage at the surface, they tend to return to their burrows to consume food. Food caches have been found within burrows, primarily composed of vegetation, suggesting they carry food in for storage. Using time-lapse photography with wildlife cameras, students in the Roles Lab have observed crayfish taking potential food items into their burrows before re-emerging. When catching this species, the Roles Lab has also experienced crayfish escaping into their burrows with the bait (worms) — on one ccasion, a single crayfish managed to escape with worms three times in one night!
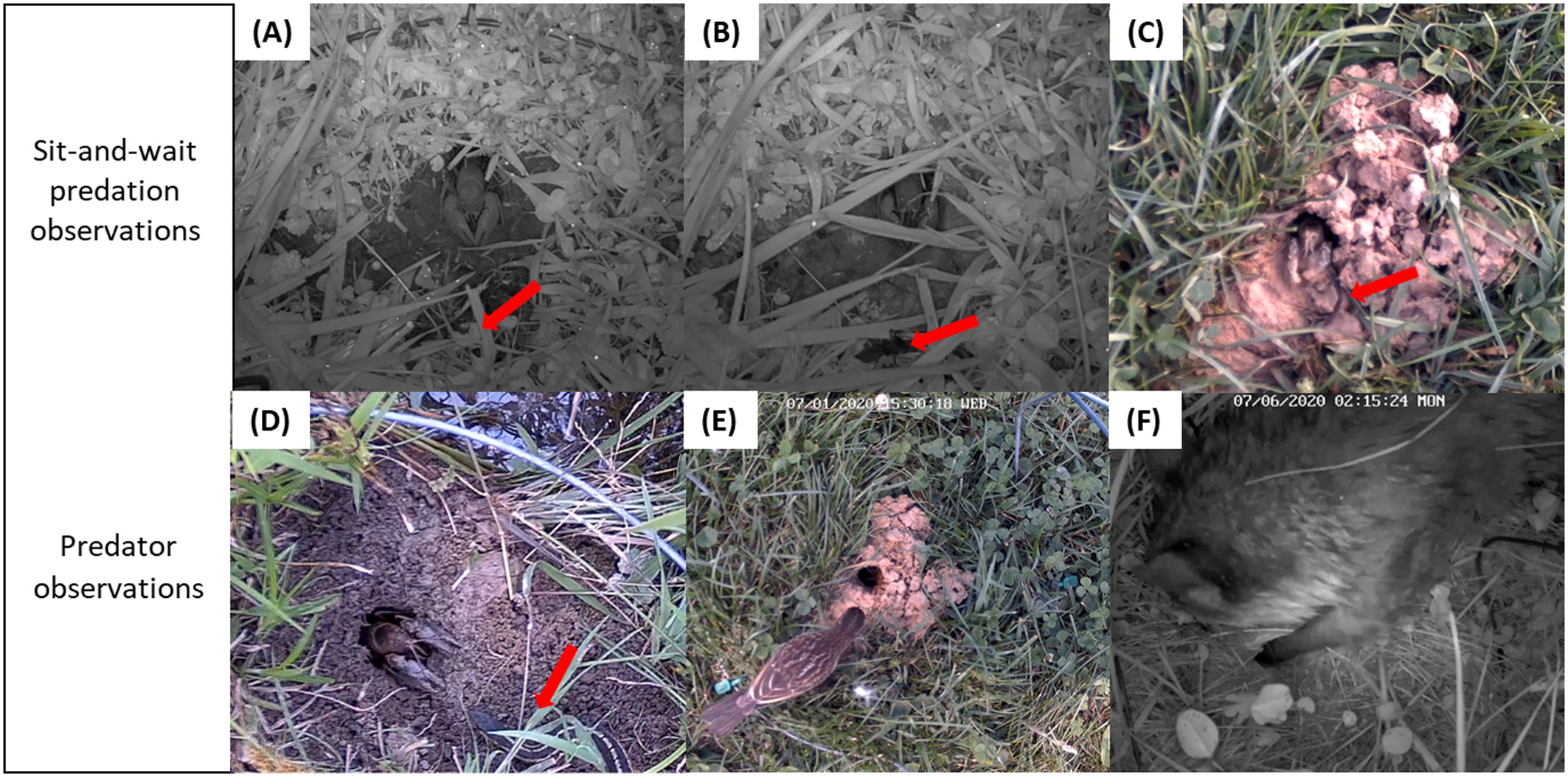
Figure 2: Video stills capture predator-prey interactions between the burrowing crayfish Lacunicambarus thomai and a (A) wolf spider, (B) dragonfly nymph, and (C) worm. The red arrows point to the prey, while the crayfish is always located above the arrows, at the entrances to their burrows. There is a tradeoff in predation for food versus being prey for other organisms when crayfish are exposed aboveground. For example, recorded visitations include those by a/an (D) eastern garter snake, (E) red-winged blackbird, and (F) raccoon. Figure 2 from Graham et al.(2022), reproduced for educational purposes.
Burrowing crayfish are primarily active at the surface at night and the Roles Lab has few observations of Creaserinus fodiens at the surface during daylight hours at this study site. If crayfish are not actively excavating, they tend to sit with most of their body in the burrow entrance, just claws and eyes peering out of the burrow. If a potential prey item approaches close to the burrow entrance, the crayfish will dart out quickly to capture it and then retreat into the burrow with its prize. When foraging, crayfish leave the burrow to gather vegetation but rarely move more than a body’s length away from the safety of the burrow, retreating to the burrow to consume or cache gathered material. Crayfish are prey for a variety of predators, including birds, snakes, frogs, and raccoons (Figure 2).
In order to examine any correlations between crayfish population
density (estimated from burrows per meter) and habitat attributes, we
should consider what aspects of the habitat might matter to the
crayfishes. Different
1A Hypothesis and predictions
We can frame the above explanations and expectations within the
context of the scientific process. Our overall
We have several potential mechanisms that could explain the expected trends and we can make the following predictions:
Vegetation type influences burrow density. We can infer that crayfish may have preference for vegetation types where their density is highest. Based on published studies, we might expect a higher burrow density in a disturbed area versus unmanaged and in a more open area compared to a woodier vegetation. Visual inspection as well as light levels can be a proxy here.
The soil properties pH, moisture, and temperature influence active burrow density. We predict soil moisture will be positively correlated with number of active burrows since crayfish require moisture. For soil pH, burrow density will be highest at neutral pH and decrease with increasing acidity. As crayfish are vulnerable to drying out in these habitats, we expect there to be a negative or non-linear (intermediate optimum) relationship between active burrow density and temperature.
Soil nutrient availability could influence vegetation type and thus also serves as a factor shaping plant community composition. We predict that soil nutrients (N, P, K) will vary with vegetation type to influence active burrow density.
Finally, as crayfish excavate burrows, the texture of the soil may be particularly important to them. Soil texture varies from loose sandy soils to heavily clay soils. We predict crayfish to prefer soils with substantial clay as those soils may be best for maintaining burrow structure as well as retaining water.
Today, we will collect data bearing on these predictions and next lab we will analyze our data to test the predictions.
1B Literature Cited
Adams, S.B., Hereford, S.G. and Hyseni, C., 2021. Burrow densities of primary burrowing crayfishes in relation to prescribed fire and mechanical vegetation treatments. Water, 13(13), p.1854. (https://doi.org/10.3390/w13131854)
Bearden, R.A., Tompkins, E.M., Weaver, C.R. and Huryn, A.D., 2022. Crayfish connections: Linking ecology and hydrogeology in Alabama’s Black Prairie using crayfish distribution patterns. Freshwater Biology, 67(4), pp.695-708. (https://doi.org/10.1111/fwb.13874)
Bloomer, C.C., Taylor, C.A., and Distefano, R.J., 2022. Integrating burrowing crayfish and waterfowl conservation management on moist-soil wetlands. Environmental Conservation, 49:130-135. (https://doi.org/10.1017/S0376892922000078)
Diehl, K.M., Storer, N.M., Wells, H.D., Davis, D.A., Loughman, Z.J. and Graham, Z.A., 2022. On the surface or down below: Field observations reveal a high degree of surface activity in a burrowing crayfish, the Little Brown Mudbug (Lacunicambarus thomai). Plos one, 17(10), p.e0273540. (https://doi.org/10.1371/journal.pone.0273540)
Graham, Z.A., Diehl, K.M., Davis, D. and Loughman, Z.J., 2022. Death from below: Sit-and-wait predatory behavior in a burrowing crayfish (Lacunicambarus thomai). Food Webs, 31, p.e00225. (https://doi.org/10.1016/j.fooweb.2022.e00225)
Rhoden, C.M., Taylor, C.A. and Peterman, W.E., 2016. Highway to heaven? Roadsides as preferred habitat for two narrowly endemic crayfish. Freshwater Science, 35(3), pp.974-983. (https://doi.org/10.1086/686919)
Seiler, S.M. and A.M. Turner. 2004. Growth and population size of crayfish in headwater streams: individual- and higher-level consequences of acidification. Freshwater Biology 49:870-881. (https://doi.org/10.1111/j.1365-2427.2004.01231.x)
2 Materials needed in the field
Make sure that your group of 4 has the following items in the field bags before we leave for the field site:
1 clipboard
field data sheets (Rite in the rain paper if raining / snowing)
1-3 pencils / pens (pencils for Rite in the rain paper)
1 sharpie (for labeling Ziploc bag)
1 meter stick
1 square meter PVC pipe transect
1 soil pH/moisture/light tester + instructions for use
1 trowel
6 plastic Ziploc bags (5 to use, one extra in case needed)
3 Methods in the field
We will work in several spots in the solar array fields, with known active crayfish burrows. These sites are also used by the Roles Lab so please avoid unnecessary damage to the site (try not to step on burrows!).
The instructor and TA will point out example active burrows when we arrive at the site. They are, essentially, mounds of dirt with holes in the middle. They often have lighter or different colored soil than the surface, due to excavations by the crayfish bringing deeper soil layers to the surface. They are considered active (and not past burrows) should the hole not be closed and no vegetation be growing over the hole; that is, the hole looks like it is being maintained.
Once your group has been given a start location by the instructor, complete the following measurements:
- A start location will be designated for each group to survey the crayfish burrows and associated habitat parameters.
- A line-transect method will be used to take data within five 1 m2 quadrats (separated by 0.5 m) along the transect. Place down the square quadrat. This is your “quadrat # 1.” Try not to step inside the quadrat (or on any burrows).
-
Within each quadrat, complete the following:
-
) Categorize the vegetation type as one of the following:
stage 1 succession = site is disturbed by regular mowing, thus hosting primarily grasses and weedy plants
stage 2 succession = grasses but also herbaceous plants, taller vegetation
-
stage 3 succession = grasses, shrubs, and small trees are present
- ) Take a soil moisture reading (takes approximately 5 minutes). Also measure soil pH and light level at the ground level.
- ) Count the number of active burrows in the quadrat.
- ) Note anything that seems ecologically relevant and is not quantified by the data that has been collected.
- ) Take a soil sample for each quadrat. Use the trowel to dig up the first 5-7 cm of topsoil. Take only one trowel-full of soil. Place the soil in a Ziploc bag. Label your samples with the date, team and quadrat numbers.
-
- Move forward (direction perpendicular to the wood chip path) about 0.5 meter and repeat step 3 until you have assessed 5 quadrats.
- If you finish early, offer help if any groups are struggling to complete their tasks or complete addi-tional quadrats to increase our sample size.
- We will leave the field together when EVERYONE has finished their measurements.
4 Methods in the lab
4A Upon return to the lab
Test the texture of your soil samples and prepare samples for nutrient analysis, using the following procedures.
Determine soil texture using the ribboning technique. Your instructor will demonstrate this technique. Use the supplied guide to perform ribboning and classify your soil samples. You can also view the guide with this LINK
For nutrient testing, we must prepare samples and then allow them to settle for a few days. Fill a clean mason jar with 5 cups of distilled water for every 1 cup of soil (for 0.5 cup soil, 2.5 cups water). Cap the jar and thoroughly mix the soil and water together via shaking for at least 1 minute. You may stir them together if shaking is not effective. Label your jar with the date of collection, your team number, and quadrat number. Set the mason jar aside to settle. Your instructors will complete the nutrient sampling before next week’s lab, after the soil has settled.
When you are finished with the soil tests, rinse all of your dirty labware with distilled water and hang to dry. Do not put soil into the sinks as it will clog the drains.
4B Data entry back at lab
Finally, after prepping your soil samples in lab, add your data to the shared Google Sheet (link below). One person should enter the data and another should verify it to be sure there are not mistakes.
We will analyze these data in our next lab. Please bring this handout to lab next week to use as a reference.
6 Pre-Lab Exercise for Lab 10
Read section 1 Burrowing Crayfish Background before answering these questions. Responses are due before the Lab 10 period.
- Most crayfish species live in surface waters (rivers and streams), a pretty different habitat from a burrow in an old field. How do you think life would be different for burrowing crayfishes in comparison to surface water crayfishes?
- Why do we think soil moisture and soil pH might matter to crayfishes? If they do matter, how will we know, how can we measure the effect?
- Why might vegetation type matter for burrowing crayfishes? Do some googling and see if you can find any information about what kinds of vegetation might be preferred by burrowing crayfishes. (You are welcome to use the papers cited in this handout.)
- What role do you think vegetation height might play in a crayfish’s choice of where to build a burrow? Remember that burrowing crayfish are mostly active at night. Explain your thinking.